Volume is the quantity characteristic specifying what space is occupied by any given substance (body). In the SI system the volume is measured in cubic meters. How it is possible to find the volume of some substance?
Instruction
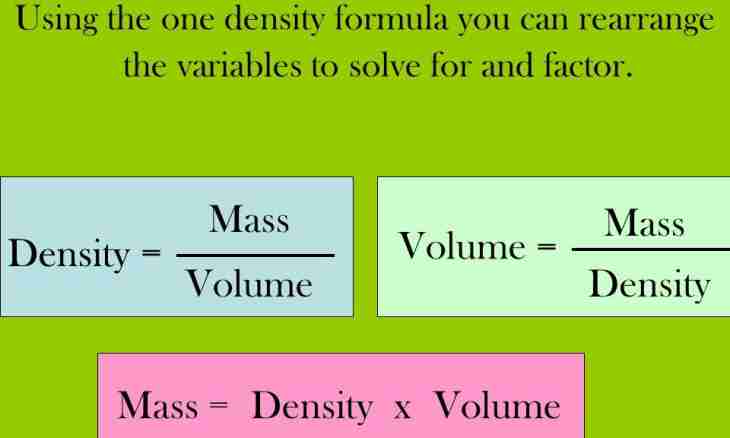
1. Simplest – if the exact mass of this substance (M) and its density is known to you (ρ). Then volume is in one action, on a formula: V = M/ρ.
2. You can use the method opened in an extreme antiquity by the great scientist Archimedes. For certain history as the Syracuse tsar Giyeron is known to you, having suspected the jeweler of fraud, ordered to Archimedes to define whether its crown is made of pure gold or cheap impurity are mixed with alloy. It would seem, everything is simple: the exact mass of a crown is known, density of pure gold is known. But before the scientist there was a task: how to determine crown volume if it is very difficult in a form? Archimedes brilliantly solved it, having weighed a crown at first in air, and then in water.
3. A difference in weight – the so-called "the pushing-out force" equal to water weight in volume of a crown. And knowing water density, it is simple to determine volume. Working by analogy, it is possible to determine the volume of any solid substance, certainly, if it is not dissolved in water and the more so does not react with it.
4. If you deal with the gas which is under the conditions close to normal, then it is very simple to determine its volume. It is only necessary to remember that one mol of any gas under such circumstances occupies volume, equal 22.4 liters. Further it is possible to make calculations, proceeding from the conditions given you.
5. For example, it is necessary to define what volume occupies 200 grams of pure nitrogen? First of all remember a formula of a molecule of nitrogen (N2) and atomic weight of nitrogen (14). Therefore, molar weight of nitrogen: 28 grams/mol. That is in 22.4 liters 28 grams of this gas would contain. And how many will be it in 200 grams? Calculate: 200х28/22.4 = 250 grams.
6. Well, and how to find gas volume if it is not under normal conditions? Here you will be come to the rescue by Mendeleyev-Klapeyrona's equation. Though it is removed for model of "ideal gas", you can quite use it.
7. Knowing such parameters necessary for you as gas pressure, its weight and temperature, you calculate volume on a formula: V = MRT/mP where R is the universal gas constant equal 8.31, m is the molar mass of gas.