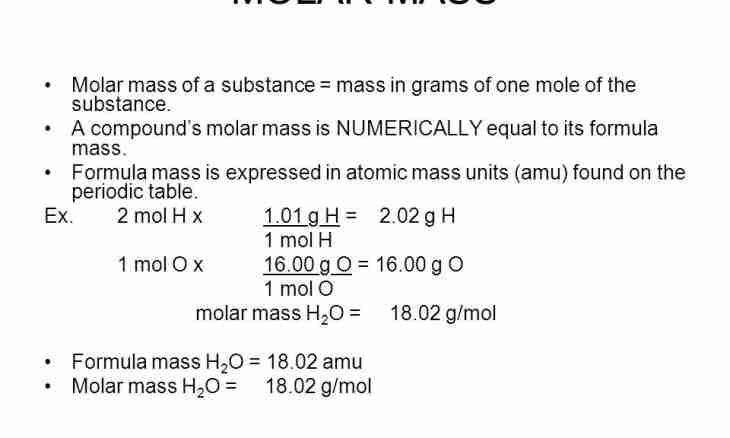
The mass of any substance, molecule is equal to the sum of mass of the atoms forming it. If when calculating to use relative atomic masses, then the relative molecular mass of substance turns out. Relative molecular weight shows how much the absolute mass of a molecule of this substance is more than 1/12 parts of the absolute mass of atom of carbon. Usually use approximate values of relative atomic and molecular masses. These sizes are dimensionless.
Instruction
1. Count quantity of elements in a molecule. For example, the molecule of H2O water consists of two atoms of hydrogen and one atom oxygen, and sulfate of iron (III) Fe2(SO4)3 contains two atoms of iron, three atoms of sulfur and twelve atoms of oxygen.
2. Count what the atomic mass of each element is equal in a molecule to. To learn the relative mass of one atom glance in the periodic system of elements. Serial number is also atomic mass. Also you can calculate it by a formula Ar (element) =m (an element)/1a.e.m. For ease of calculations use approximate value.Ar (H)=1? 2=2; Ar(O)=16? 1=16Ar (Fe)=56? 2=112; Ar(S)=32? 3=96; Ar(O)=16? 12=192
3. Put the received results. It will also be the molecular mass of substance.Mr (H2O) =2Ar (H)+Ar(O) =2+16=18Mr (Fe2(SO4)3) =2Ar (Fe) +3Ar (S) +12Ar (O)=112+96+192=400
4. Except relative molecular weight when calculating use molar weight more often. Its unit of measure - g/mol. It is in number equal to the relative molecular mass of substance.M (H2O)=18 of / мольM (Fe2(SO4) of 3=400 g/mol