Valency of chemical element is an ability of atom to attach or replace a certain number of other atoms or atomic groups with formation of a chemical bond. It is necessary to remember that some atoms of the same chemical element can have different valency in different connections.
It is required to you
- Mendeleyev's table
Instruction
1. Hydrogen and oxygen are considered to be monovalent and bivalent elements respectively. A measure of valency is the number of atoms of hydrogen or oxygen which the element attaches for formation of hydride or oxide. Let X - an element which valency needs to be determined. Then XHn is hydride of this element, and XmOn is its oxide. Example: an ammonia formula - NH3, here at nitrogen valency 3. Sodium odnovalenten in the Na2O connection.
2. For determination of valency of an element it is necessary to increase amount of atoms of hydrogen or oxygen in connection by the valency of hydrogen and oxygen respectively, and then to divide into number of atoms of chemical element which valency is.
3. Valency of an element can be determined also by other atoms with the known valency. In various connections atoms of the same element can show various valencies. For example, the dvukhvalentna in the H2S and CuS connections, a chetyrekhvalentna in SO2 and SF4 connections, a shestivalentna in SO3 and SF6 connections is gray.
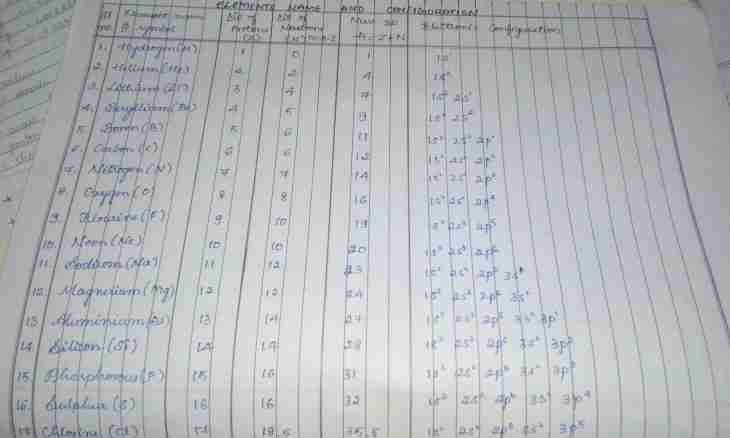
4. The maximum valency of an element is considered equal to number of electrons in an outer electron shell of atom. The maximum valency of elements of the same group of a periodic system usually corresponds to its serial number. For example, the maximum valency of atom of carbon C has to be equal 4.