There are two ways of definition of a pH value of solutions – electrometric (by means of rn-meter) and colorimetric (by means of chemical indicators). The first respectively is more exact and allows to define acidity in any environments, any structure, color and consistence whereas the second method suits for transparent water solutions. This method of definition rn solutions is based on use of the acid and main indicators which coloring changes with change of acidity of the environment.
It is required to you
- Mix of equal volumes of acids of 0.12 N: phosphoric, acetic, boric.
- Caustic rubbed NaOH, 0.2 N.
- Indicators:
- Tropeolin 00, 0.1% water solution.
- Methyl orange, 0.1% water solution.
- Methyl red, 0.1% solution in 60% alcohol.
- Bromtimolovy blue, 0.05% solution in 20% alcohol.
- Krezolovy red, 0.04% water solution.
- Phenolphthalein, 0.1% spirit solution.
- Thymolphthalein, 0.1% spirit solution.
- Table of transitions of tsvetovindikator
- Table of the acid and main indicators.
Instruction
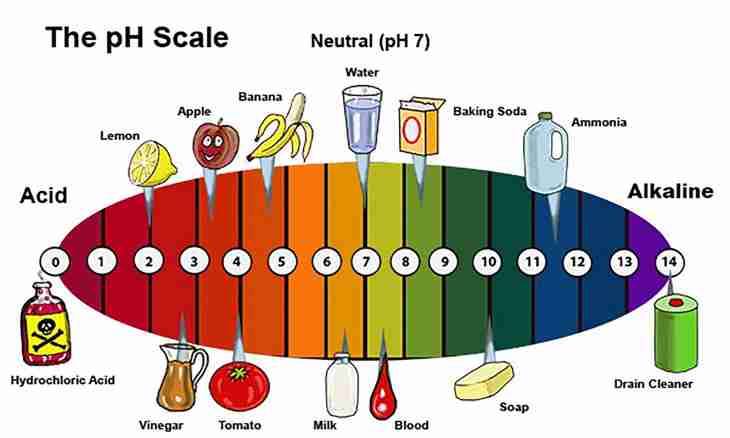
1. Carry out approximate determination of value rn test solution by means of the universal indicator or universal indicator paper. Degree of acidity of water solution is expressed by a pH value (quantity of ions of hydrogen) which size fluctuates from 0 (extremely high acidity) up to 14 (extremely high alkalinity). At the same time to change of concentration of hydrogen ions by 10 times there corresponds change rn on one unit. The neutral environment has ph indicator equal 7 (at the room temperature). Methyl orange at rn <3.1 has red color, and at rn> 4.4 – yellow; litmus at rn <5 red, and at rn> 8 – blue. For example, the litmus piece of paper gained red color from you, therefore, test solution has the increased acidity, value rn less than 5.
2. Find indicators which diagnose the area of acidity which is already determined by the universal indicator more precisely in the table. I.e. you look at what indicator in the field of values the pH value from 0 to 5 is observed – at methylene red, methyl orange and a tropeolin 00.
3. Prepare a standard number of the buffer solutions covering this area rn. For this purpose take the dry calibrated test tubes, place in them 5 ml of mix of acids, a caustic natr put in each test tube according to the table. Number (or somehow differently mark) them. Mix solutions and the pipette remove superfluous, having brought solution volume in each test tube exactly to 5 ml.
4. In a separate clean test tube (if you check several indicators at once, take test tubes on number of indicators) gain 5 ml of test solution. Add 2 drops of the necessary indicator and compare coloring of test solution to coloring of solutions of a standard row.