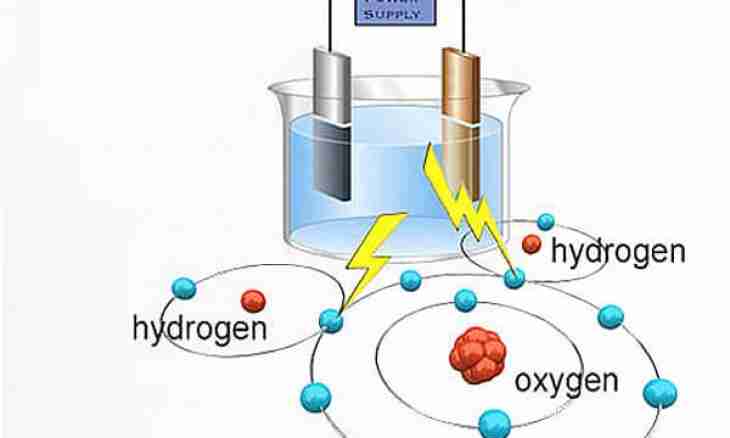
Oxygen can be emitted from many chemical compounds. For the industrial purposes oxygen is received most often by liquefaction of air with simultaneous cleaning. But oxygen can be received also from water. However, in house conditions or in school laboratory it it can turn out very little. For this purpose it is necessary to divide a water molecule into atoms of oxygen and hydrogen.
It is required to you
- - water;
- - sulfuric acid;
- - DC power source of 6-12 V;
- - galvanic can (rectangular glass vessel of 5-8 l);
- - coal electrodes from the electric battery;
- - 2 transparent plastic glasses;
- - bitumen;
- - tube from a dropper;
- - test tube;
- - glass jar;
- - soldering iron;
- - 2 wires.
Instruction
1. Take a plastic glass. Make an opening in its bottom and insert into it an electrode so that he settled down coal in a glass. Isolate a junction of an electrode and a glass bitumen from a bottom. In the same way process also the second glass for the second electrode. Solder a wire to a metal part of each electrode. It is better to take wires of different color, for example, red and blue.
2. In a galvanic bathtub fill in water approximately on 2/3 heights. Add 1-2 ml of the divorced sulfuric acid there. Concentration is not of great importance as sulfuric acid is necessary only for water polarization.
3. Install glasses with electrodes so that electrodes were shipped in water, and the quantity of air between a water surface and the bottom of a glass whenever possible was minimum. Connect electrodes to current source terminals. For example, connect a red wire to the anode, and blue — to the cathode. Through transparent walls of a galvanic bathtub and glasses observe how about electrodes bubbles which rise up begin to be formed and accumulate in glasses. There is the following reaction: 2(H2O) →2H2+O2. About the cathode (negative electrode) hydrogen molecules, and about the anode — an oxygen molecule accumulate.
4. By means of a tube from a dropper it is possible to take away any given gas in bank with water and to fill with its help a test tube for the analysis. For example, in oxygen it is possible to burn red-hot the made metal wire. Hydrogen burns. It is necessary to remember that during the experiment it is necessary to avoid mixing of these gases and also mixing of hydrogen with air.
5. The amount of oxygen received at this experiment is small because he actively enters interaction with a coal electrode and is absorbed by it, in addition forming carbon dioxide as impurity. Obtaining bigger amount of oxygen requires the inert anode. Such anode can be manufactured of a plate of platinum or of a metal plate, having covered it with a layer of gold or palladium.