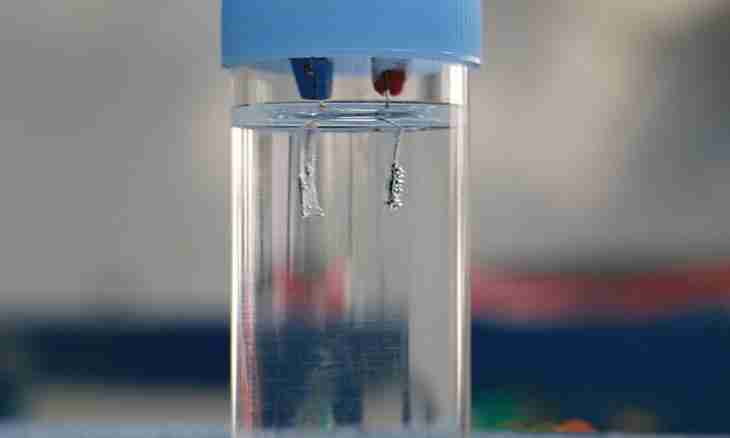
Very often in chemistry there are situations when it is required to separate several chemical elements. Quite often it is necessary to separate oxygen and hydrogen, for example for obtaining energy.
Use of the electrolyzer
It becomes by means of the special device of the electrolyzer. It represents a tube in which there is an alkali. In it there are also couple of nickel electrodes. The principle of polarity is the cornerstone. During work, oxygen will go to that part of a pipe where the positively charged pole of an electrode is located, and hydrogen will aspire in the opposite direction to a negative pole. This method of receiving O2 and H2 is suitable more for laboratories. Besides, it is not calculated on large volumes of receiving gases.
Application of an electrolytic bathtub
Bathtubs are suitable for obtaining a large amount of hydrogen and oxygen. They are used at the large plants. The bathtub represents the tank filled with liquid which it is capable to pass current. In it there are several electrodes. They settle down in parallel on the relation to each other. Depending on it bathtubs can be monopolar and bipolar.
In the first option a part of electrodes is connected to a positive pole of current, and the others - to negative. Mechanism of formation of these two gases following: when passing direct electric current through electrolyte between electrodes there is a release of gases. In order that they did not mix up, two pipes are brought to a bathtub. To one of them there is an oxygen, and to another - hydrogen. There are several ways of how to isolate each electrode. It is possible to make it by means of special bells. They are made of metal. As a result of chemical interaction of electrolyte with current on electrodes vials of gas which begin to rise are formed. By means of bells their division is provided, and each of gases goes to the pipe subsequently. There is also the second method which is based on use of special partitions. As those various materials which do not pass gas can be applied. Thickness of such partition is about 2 mm. It provides isolation of both electrodes. After gases came to the system of pipes, they move to special rooms. Fill with these gases big cylinders. At the same time it is necessary to create the optimum pressure which has to be 150 atmospheres. In such type of O2 and H2 can be transported to the consumer. Similar gases are in pure form very actively applied now. Owing to all this it is possible to draw a conclusion that the separation of hydrogen from O2 is carried out in the presence of the equipment by electrolysis.