Diamond is the mineral relating to one of allotropic modifications of carbon. Its distinctive feature is the high hardness which by right brings it a rank of the most solid substance. Diamond rather rare mineral, but together with it and the most widespread. Its exclusive hardness finds the application in mechanical engineering and the industry.
Instruction
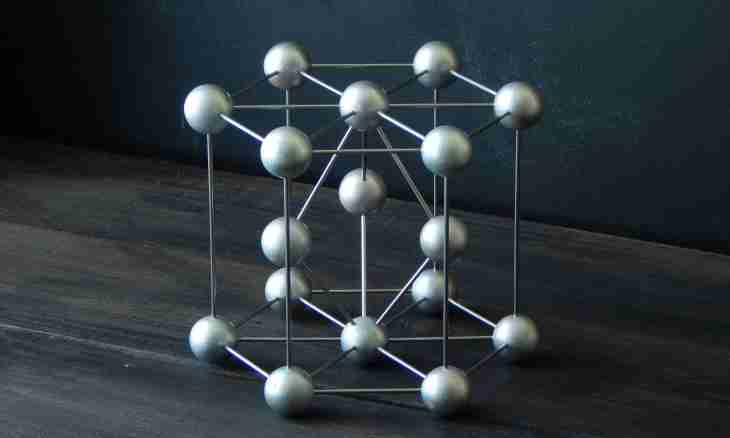
1. Diamond has an atomic crystal lattice. The carbon atoms forming the molecule basis are located in the form of a tetrahedron thanks to what diamond has such high durability. All atoms are connected by strong covalent communications which are formed, proceeding from the electronic structure of a molecule.
2. Atom of carbon has sp3-hybridization of orbitals which are located at an angle in 109 degrees and 28 minutes. Overshoot of hybrid orbitals happens in a straight line in the horizontal plane.
3. Thus, at overshoot of orbitals under such corner the aligned tetrahedron which belongs to a cubic system therefore it is possible to tell that diamond has cubic structure is formed. Such structure is considered one of the strongest in the nature. All tetrahedrons form three-dimensional network of layers of six-membered rings of atoms. Such steady network of covalent communications and their three-dimensional distribution conducts to the additional durability of a crystal lattice.
4. Crystal lattice at diamond rather difficult. It consists of two simple sublattices. The area of space lying closer to this atom, than to other atoms for a lattice of diamond represents triakis the truncated tetrahedron. Such type of a lattice silicon, germaniye also tin, mainly an alpha form possess.
5. Triakisov the truncated tetrahedron represents the polyhedron made of four hexagons and twelve isosceles triangles. It can be used for a tesselyation of three-dimensional space. As an example of a tesselyation it is possible to consider a square which needs to be cut on diagonal, that is to tesselirovat a square on two triangles. Tesselyation in itself improves realism of three-dimensional model, and in relation to a crystal lattice of diamond does it to more realistic.
6. At the moment the science came to receiving diamonds in the synthetic way. For synthesis of such crystals use, as a rule, high-carbon alloy of nickel with manganese or the high-frequency plasma concentrated on a substrate where diamond is formed. When receiving mineral in such a way, its crystal lattice strongly differs from a lattice which natural diamond has. There is a shift of layers of carbon in this connection they are located chaotically. For this reason the crystals received in such a way have the smaller durability and rather high fragility.