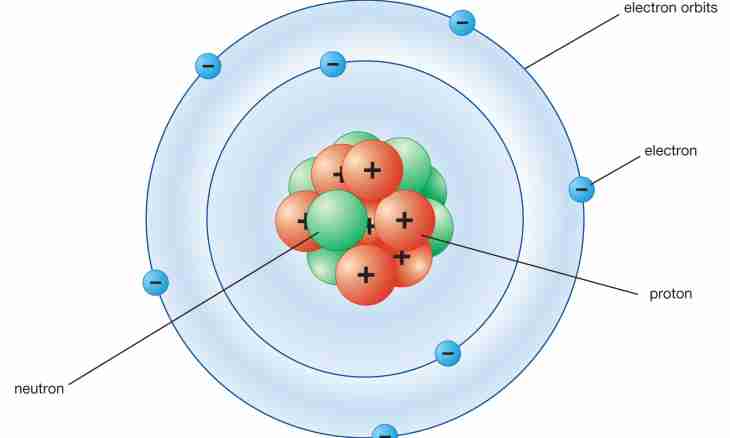
Better to understand what is isotopes, it is possible to play. Present big transparent spheres. They sometimes can be seen in the park. Each sphere is an atomic nucleus.
Each kernel consists of protons and neutrons. Protons are positively charged particles. Instead of protons you will have toy hares on batteries. And instead of neutrons - hares without batteries, they do not bear any charge. Put in both spheres on 8 hares with batteries. Means, in each sphere kernel at you on 8 positively charged protons. Now here what it is necessary to make with hares without batteries – neutrons. In one sphere put 8 hares neutrons, and in another –7 hares neutrons. The mass number is the sum of protons and neutrons. Count hares in each sphere and learn mass number. In one sphere mass number – 16, in other sphere – 17. You see two identical kernels spheres with the same number of protons. The number of neutrons at them differs. Spheres acted as isotopes. Do you know why? Because isotopes are options of atoms of one element with different number of neutrons. It appears, these spheres actually not just atomic nuclei, and the most real chemical elements in Mendeleyev's table. Remember what chemical element has a charge +8? Of course, it is oxygen. Now it is clear that at oxygen it is several isotopes, and all of them differ from each other in number of neutrons. Oxygen isotope with mass number 16 has 8 neutrons, and oxygen isotope with mass number 17 has 9 neutrons. The mass number is specified from above to the left of a chemical symbol of an element.
Present spheres with hares, and it will be easier to understand scientific definition of isotopes. So, isotopes are atoms of chemical element with an identical charge of a kernel, but different mass number. Or such definition: isotopes are options of one chemical element which take one place in the periodic system of elements of Mendeleyev, but at the same time differ in the mass of atoms. Why knowledge of isotopes is necessary? Isotopes of different elements are applied in science, medicine. A specific place is held by hydrogen isotope – a deuterium. Important connection of a deuterium - heavy D2O water. It is applied as the delay mechanism of neutrons in nuclear reactors. Isotopes of a pine forest are used in atomic science and technology, carbon isotopes – in medicine. Isotopes of silicon will help to increase the speed of computation processes in computers.