Not all remember from a chemistry course what is monomer and what role it plays in everyday life. Actually, monomers have the major impact on the world around and participate in education of many connections necessary today.
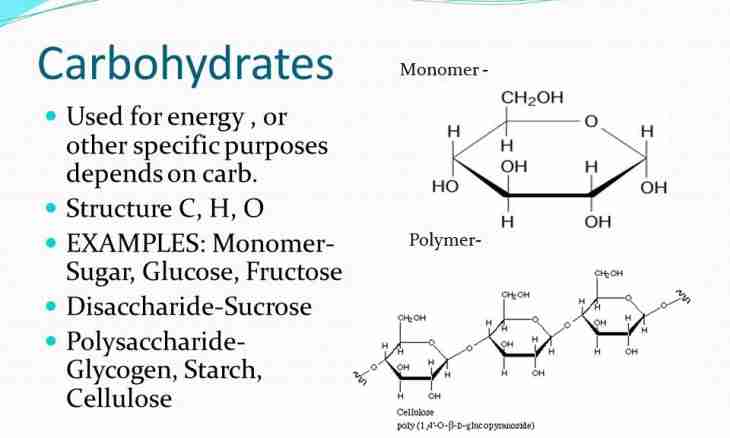
Monomer (from Greek "mono" - one and "мерос" "part") is atom or a small molecule which can form polymeric communications. Also monomers often call monomeric links as a part of polymeric molecules. The most widespread natural monomer is glucose which forms such polymers as cellulose and starch, and makes more than 76% of mass of all plants. Most often the term "monomer" belongs to organic molecules which form such synthetic polymers as, for example, blamed chloride which is used for production polymer polyvinyl of chloride (PVC). It is possible to carry molecules of nonsaturated hydrocarbons to other organic monomers - alkenes and alkynes.
Amino acids are natural monomers which at polymerization form proteinaceous connections. Nucleotides (the monomers located in a cage kernel) will be polymerized with formation of nucleinic acids - DNA and RNA. The isoprene is natural monomer and will be polymerized in the form of natural rubber. In the industry acrylic monomers in the form of acrylic acid, acrylamide are also widely used.
Monomers differ on functionality. They can be bifunctional if have two functional groups, trifunktsionalny - if three, etc. Connections with lower molecular weight are constructed of the monomers which are also called dimeasures, trimmers, tetramers, pentameasures, octameasures, etc. if they, respectively, have 2, 3, 4, 5, 8 and more monomeric units. Any quantity of these links can be designated by the corresponding Greek prefix, for example decameasures is formed of 10 monomers. Large numbers are often written in English instead of Greek. The molecules increasing a small number of monomeric units to several dozen are called oligomers.