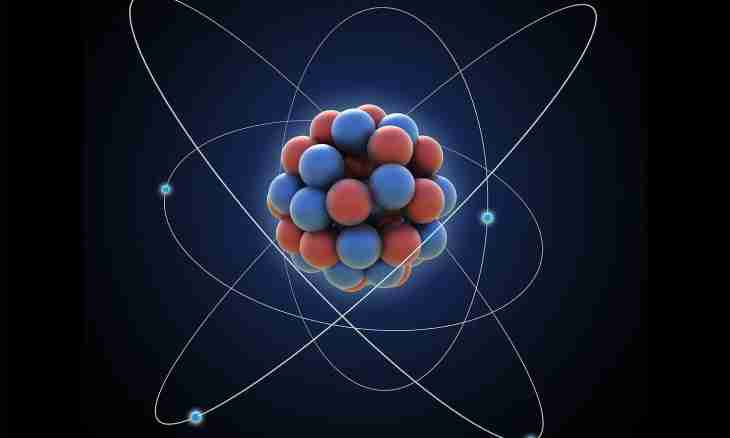
Atom consists of a kernel and electrons. In a kernel practically all mass of atom is concluded, but it borrows only is insignificant a small part of its volume. Electrons rotate around a kernel on circular and elliptic orbits, forming an electron shell. Such structure of atom was confirmed with experiments of the scientist Rutherford studying a deviation of particles when passing X-rays through the thinnest plates of gold. Each electron bears a single negative charge. Why atom how confirm researches, is neutral?
Atom is considered neutral as its kernel consists of particles: protons and neutrons. Each proton though it is much heavier than an electron (by 1836 times), it bears a single charge too. Only not negative, but positive. The neutron as it is easily possible to understand from the name, does not bear in general any charge: neither positive, nor negative. The simplest example – atom of hydrogen, the first element of the Table of Mendeleyev. The atomic nucleus of its isotope of protium (most widespread) consists of the only proton. Respectively, around it the only electron rotates on a circular orbit. Their charges mutually counterbalance each other, and atom of protium is neutral. Hydrogen has also other isotopes: a deuterium (in which structure of a kernel, besides a proton one neutron enters) and tritium (its kernel contains a proton and two neutrons). These isotopes differ in properties from protium a little, but too are neutral. To any element of the Table of Mendeleyev there corresponds the serial number. It coincides with quantity of protons in its kernel. So, at silicon (Si) – 14 protons, at manganese (Mn) – 25 protons, at gold (Au) – 79 protons. Respectively, the kernel of each atom of these elements "attracts" to itself 14, 25 and 79 electrons, forcing to rotate on circular and elliptic orbits. And atoms are neutral as negative charges are counterbalanced by positive charges. Whether always atoms keep neutrality? No, it is very frequent they, having entered a chemical bond with other atoms, or attract to themselves others electron, or concede the. It depends on so-called degree of electronegativity. If atom attracted an excess electron, it becomes a negatively charged ion. If conceded the electron – too becomes an ion, but already positively charged.