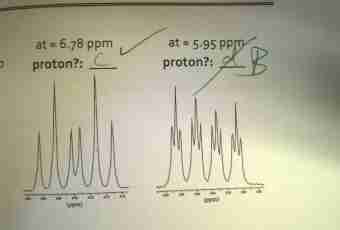
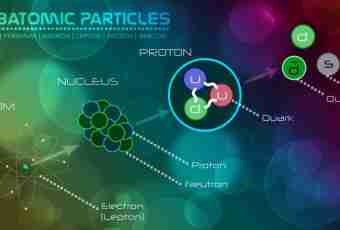
Chemistry — the exact science therefore when mixing various substances it is just necessary to know their accurate proportions. For this purpose it is necessary to be able to find the mass of substance. It is possible to make it in various ways, depending on what sizes are known to you.

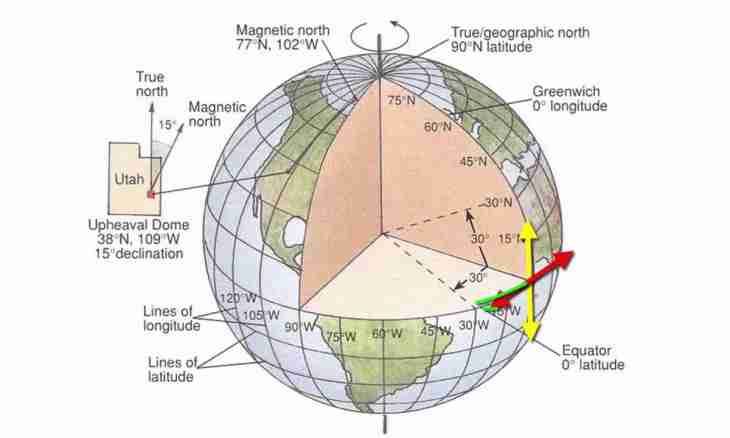
Inertia does not come down only to its mechanical manifestations. All real surely resists any influences, otherwise the world will not be able to exist. Visible manifestations of inertia can not be, but it does not vanish anywhere and never.