Hybridization in chemical sense of the word is a change of a form and energy of electronic orbitals. This process happens when the electrons belonging to different types of communication take part in formation of communication.
Instruction
1. Consider a molecule of the most plain saturated hydrocarbon of methane. Its formula looks as follows: CH4. The spatial model of a molecule represents itself a tetrahedron. Atom of carbon forms with four atoms of hydrogen absolutely identical on length and binding energy. According to the above-stated example, 3-P electrons and 1 S – an electron which orbital began to correspond in accuracy to orbitals of three other electrons as a result of the happened hybridization participate in them. Such type of hybridization is called sp^3 hybridization. It is inherent in all saturated hydrocarbons.
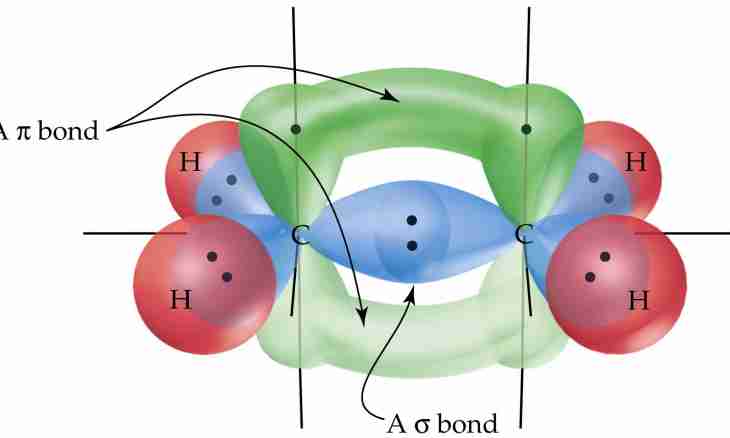
2. And here the most ordinary representative of unsaturated hydrocarbons – ethylene. Its formula looks as follows: C2H4. What type of hybridization is inherent in carbon in a molecule of this substance? As a result of it three orbitals in the form of the asymmetrical "eights" lying in one plane at an angle 120^0 to each other are formed. They were formed by 1-S and 2-P electrons. The last the 3rd P – the electron did not alter the orbital, that is it remained in the form of correct "eight". Such type of hybridization is called by sp^2 hybridization.
3. How communications in an ethylene molecule are formed? Two gibridizovanny orbitals of each atom entered interaction with two atoms of hydrogen. The third gibridizovanny orbital formed communication with the same orbital of other atom of carbon. And remained P – orbitals? They "were attracted" to each other on both sides from the molecule plane. Between atoms of carbon the double communication was formed. sp^2 hybridization is inherent in atoms with "double" communication.
4. And what occurs in a molecule of acetylene or an etin? Its formula looks as follows: C2H2. In each atom of carbon of hybridization only two electrons are exposed: 1 - S and 1 – R. Ostalnye two kept orbitals in the form of the "correct eights" which are blocked" in the plane of a molecule and on both sides from it. Here therefore such type of hybridization carries the name sp – hybridizations. It is inherent in atoms with threefold communication.