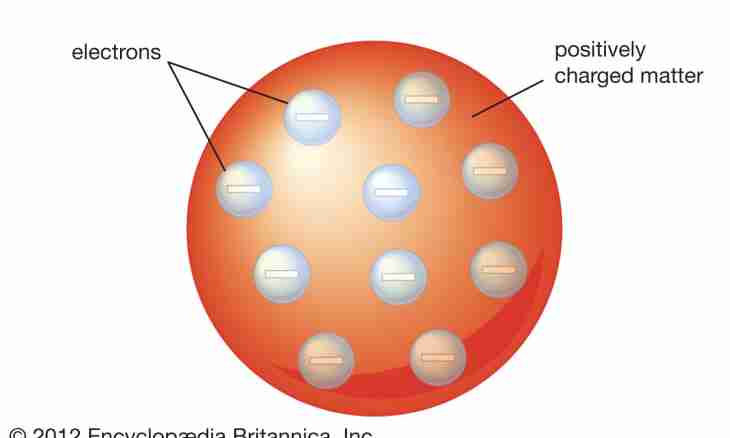
Atom of any chemical element consists of an atomic nucleus and, addressing around it, electrons. And of what the atomic nucleus consists? In 1932 it was established that the atomic nucleus consists of protons and neutrons.
It is required to you
- - periodic table of chemical elements of D.I. Mendeleyev.
Instruction
1. The proton represents a positively charged particle with a weight exceeding the mass of an electron by 1836 times. Electric charge of a proton coincides on the module with an electron charge, so, the charge of a proton is equal 1.6*10 ^ (-19) Pendant. Kernels of different atoms contain different number of protons. For example, in a hydrogen atomic nucleus only one proton, and in a gold atomic nucleus – seventy nine. The number of protons in a kernel coincides with serial number of this element in D.I. Mendeleyev's table. Therefore to define number of protons in a kernel of chemical element, it is necessary to take Mendeleyev's table, to find in it the necessary element. The integer specified above is serial number of an element - it is and there is a number of protons in a kernel. Primer1. Let it is necessary to define number of protons in a polonium atomic nucleus. Find in Mendeleyev's table chemical element polonium, it is located at number 84, means in its kernel there are 84 protons.
2. It is interesting that the quantity of protons in a kernel coincides with number of the electrons moving around a kernel. That is the number of electrons in atom of an element is defined as well as number of protons – serial number of an element. Example 2. If serial number of polonium - 84, then in it 84 protons (in a kernel) and as much - 84 electrons.
3. The neutron represents not charged particle with a weight which more than the weight of an electron by 1839 times. Besides serial number, in the periodic table of chemical elements for each substance one more number which if to round it, shows total number of particles (protons and neutrons) in an atomic nucleus is specified. This number is called mass number. For determination of quantity of neutrons in a kernel it is necessary to subtract quantity of protons from mass number. Example 3. Quantity of protons in polonium atom – 84. Its mass number is equal 210, so for definition of number of neutrons find the difference of mass number and serial number: 210 – 84 = 126.