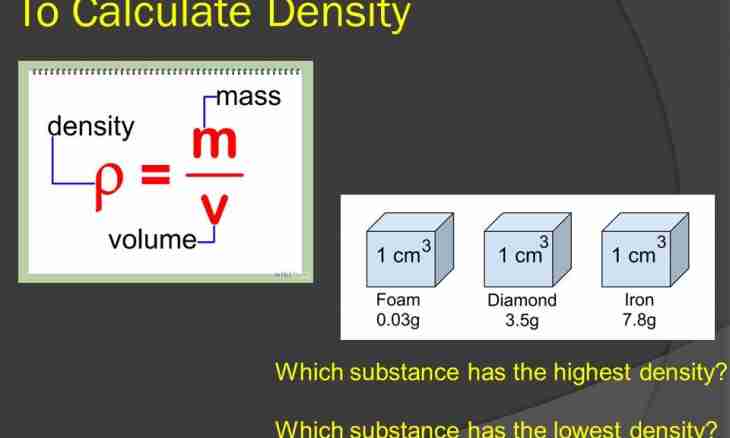
The objects made of different substances have different masses. The physical quantity showing what the mass of substance is equal in unit of volume to is called substance density. Density unit in the International system of units is the kilogram divided into cubic meter. In practice often this size is measured in the grams divided into centimeter cubic. Density of the same substance in strong, liquid and gaseous state is various. Density is a table value, that is almost all values for various substances which are in different aggregate states are already counted and placed in special tables. But if there is no such table near at hand, it is possible to calculate independently density of this substance.
Instruction
1. To calculate density of substance it is necessary to know the weight of body which is made of this material. Most often weight can be expressed in grams or kilograms, a transfer needs to be made in those units in which required size – density will be measured. For establishment of communication between units the rule is considered: 1 g = 0.001 kg, 1 kg = 1000. Example: 5 kg = 5000 g; 346 g = 0.346 kg.
2. The following size determining density of substance is a volume of a body. This size geometrical, is actually equal to the work of three sizes of the studied subject: heights, width and lengths. Volume is measured in cubic meters or centimeters, that is sizes in the third degree. So, 1 centimeter is cubed equal to one million part of cubic meters.
3. Knowing two above-stated sizes, it is possible to write down a formula for calculation of density of substance: density = weight/volume, from here also turns out unit of measure of required size. Example. It is known that the ice floe of 2 cubic meters has weight of 1800 kg. To find ice density. Decision: density is equal to 1800 kg / 2 meter cubed, 900 kg divided into cubic meters turn out. Sometimes to have to transfer density units each other. Not to get confused, it is necessary to remember: the 1g/cm is cubed equal to 1000 kg/m cubed. Example: 5.6 g/cm it is cubed equal 5.6*1000 = 5600 kg/m cubed.