It is possible to determine the volume of any capacity in several ways. Geometrically it can be done, if capacity possesses the correct form. If the vessel is hermetically closed, but it is known of what material its walls are made, its volume can be calculated. For measurement of volumes of tanks of irregular shape it is possible to use liquid or gas.
It is required to you
- - formulas for definition of solids;
- - measured vessel or capacity of the correct form;
- - gas of the known weight.
Instruction
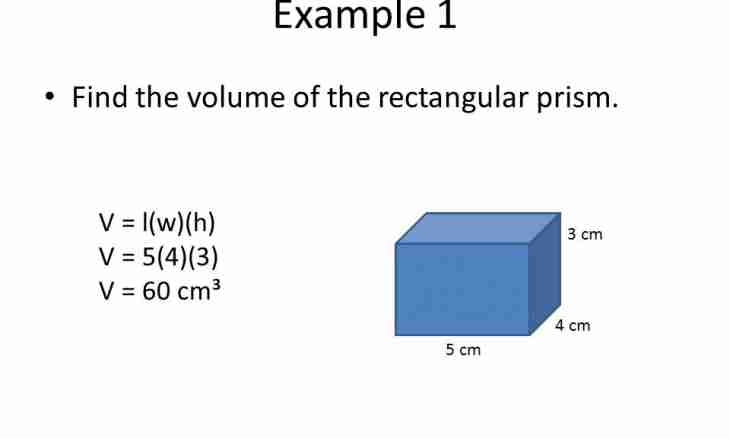
1. If capacity has the correct geometrical form (parallelepiped, a prism, a pyramid, a sphere, a cylinder, a cone, etc.), measure its internal linear dimensions and make calculation. For example, if the barrel has the cylinder form, measure its internal diameter by d and to a visot of h. Then calculate volume by a formula for scoping of a cylinder. For this purpose π ≈ 3.14 increase number by a square of diameter of the basis and height of a barrel, and result divide into number 4 (V=π ∙ d² ∙ h/4). For other solids use the corresponding formulas of volumes too.
2. In case it is difficult to calculate volume, because of a capacity form, fill in capacity with liquid (water) so that it completely filled it. In this case, the volume of water will be equal to the volume of the measured capacity. Then accurately drain water in a separate vessel. It can be a special measuring cylinder with divisions, or the capacity of geometrically correct form. If water is filled in in a measuring cylinder or other vessel, determine liquid volume by its scale. It will be equal to required size for the measured capacity. If water is filled in in the capacity of the correct form, calculate its volume by the technique stated in the previous point.
3. Sometimes capacity is too high in order that it was possible to use liquid. In this case, begin to rock in it the known mass of gas (it is possible only if it can be closed hermetically), with the known molar mass, for example, of kg/mol M=0.028 nitrogen. After that measure pressure by the manometer and temperature the thermometer in capacity. Express pressure in Pascals, and temperature in Kelvins. Determine the volume of the pumped gas. For this purpose the mass of gas m increase by its temperature T and universal gas constant R. Divide result into the molar mass of M and pressure P (V=(m∙R∙T)/(M∙P). Receive result in m³.