Discovery of atom became the first step on the way of knowledge of a microcosm. It occurred only at the end of the 19th century in spite of the fact that existence of atom was predicted still by Ancient Greek scientists.
150 years ago scientists believed that the atoms which are a part of all substances are indivisible by the nature. The modern science showed long ago that it not so. Everything began with opening of an electron.
Opening of an electron
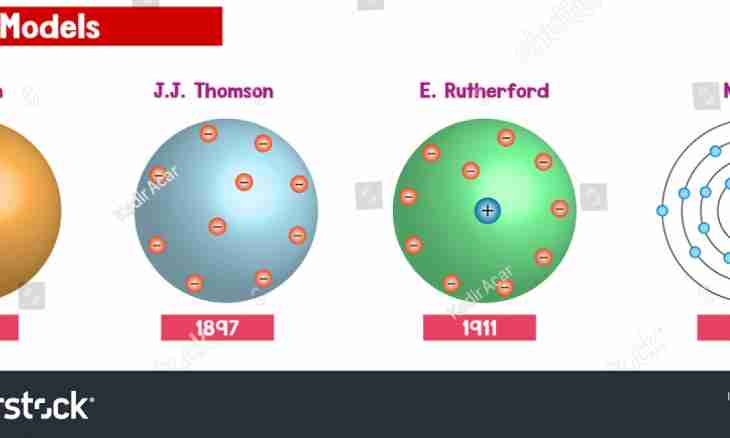
At the end of the 19th century in science of that time there was the real revolution. The famous scientist J.J. Thomson (lord Calvin) opened an electron, the microparticle possessing a negative charge. According to its theory, electrons are present at each atom. The lack of the necessary equipment did not allow to define precisely how these particles in atom are located and whether they move. Physicists could only indulge in philosophical reasonings on this subject.
Lord Calvin also offered the first model of atom. According to its model, atom represents a particle of positively charged substance in which there are electrons. Many compare such atom to cake in which highlights are interspersed.
Rutherford experiments
The English physicist Ernest Rutherford researched atom too. Its experiments destroyed one of postulates of physics of a microcosm of that time. This postulate was that atom - an indivisible particle of substance. By then the natural radioactivity of some chemical elements was already open. Rutherford also used one of them for experience. Results of an experiment allowed to create new model of atom. Rutherford irradiated a gold foil with alpha particles. It turned out so that some of them could pass freely through a foil, and some dissipated under different corners. If atoms of gold had the structure offered by Thomson, the alpha particle which has quite large diameter could only be reflected at right angle. Thomson's model appeared incapable to explain this phenomenon therefore Rutherford offered the model which he called planetary. According to it, atom represents a kernel around which electrons rotate. It is possible to give analogy to the Solar system: planets rotate around the Sun. Electrons move on own orbits.
Quantum theory of Bohr
The planetary model of atom was well coordinated with many experiments, but it could not explain long existence of atom. It is all about outdated classical ideas of atom. An electron, moving on an orbit, has to radiate (to give) energy. Through small time (about 0.00000001 sec.) it has to fall to atom owing to what existence of the last will stop. But why then all of us still exist and did not break up to the smallest particles? The answer to this question was given by the quantum theory of Bohr. Today there is a set of models of atom and an atomic nucleus. Each of them has the shortcomings and advantages. The mankind will never be able to create perfect model which would explain the surprising phenomena occurring in it.