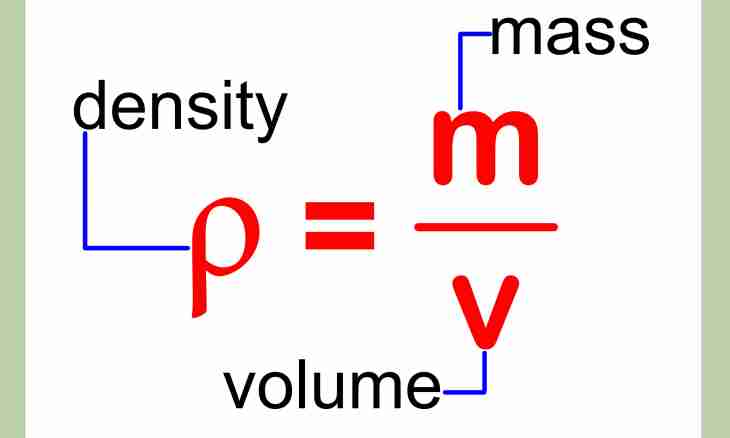In chemical laboratories and when conducting chemical experiments in house conditions often happens it is necessary to determine the relative density of any given substance. Relative density represents the relation of density of concrete substance to density of another under certain conditions or to density of reference substance to which the distilled water is accepted. Relative density is expressed by abstract number.
It is required to you
- - tables and reference books;
- - areometer, densimeter or special scales.
Instruction
1. Determine the relative density of substances in relation to density of the distilled water by a formula: d=p/p0 where d is required relative density, p is density of the studied substance, p0 is density of reference substance. The last parameter tabular is also determined quite precisely: at 20 OS the distilled water has density of 998.203 kg/CBM, and it reaches the maximum density at 4 OS – 999.973 kg/CBM. Before calculations do not forget that r and r0 have to be expressed in identical units of measure.
2. Besides, the relative density of substance can be learned in physical and chemical reference books. Numerical value of relative density always to equally relative specific weight of the same substance in the same conditions. Conclusion: use tables of relative specific weight the same as if it were tables of relative density.
3. Determining relative density, always consider temperature of the studied and reference substance. The fact is that density of substances decreases when heating and increases when cooling. If temperature of the studied substance differs from standard temperature, make the amendment. Calculate it as average change of relative density on 1 wasps. Look for the necessary data on nomograms of temperature amendments.
4. For fast calculation of relative density of liquids in practice use the areometer. For measurement of relative density of gases and solids use densimeters and special scales. The classical areometer is the glass tube extending in the lower part. On the lower end of a tube there is a tank with fraction or special substance. On the top part of a tube the divisions showing numerical value of relative density of the studied substance are put. Many areometers in addition equip with thermometers for measurements of temperature of the studied substance.
5. Ship the areometer in the studied liquid or solution. The relative density of the studied liquid will be lower, the more deeply the areometer under the influence of its body weight will plunge into it. Therefore when carrying out measurements the device should not be held with a hand. Having waited when the surface of substance calms down and the areometer will stand, recognize the relative density of the studied liquid by divisions of a scale.