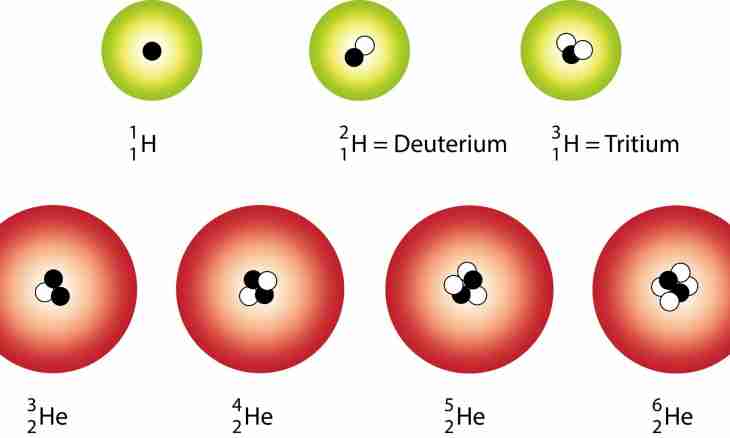
The atomic nucleus consists of particles with the general name nucleons. Two of their views – neutrons and protons are known. The quantity of neutrons can be found on the mass of atom as it is almost equal to the mass of an atomic nucleus (the mass of an electron shell is negligible) and its charge.
It is required to you
- - periodic table of chemical elements (Mendeleyev's table);
- - proton charge;
- - chemical elements.
Instruction
1. Each atom of substance is described in the Periodic Table. Find the element cell corresponding to the studied atom. Find its relative atomic mass in the lower part of a cell. If it is presented by fractional number, round it to whole (it will be the relative atomic mass of the isotope, most widespread in the nature). This number reflects quantity of nucleons in an atomic nucleus. Find serial number of the studied chemical element. It is equal to number of protons in a kernel. Define quantity of neutrons, having taken away quantity of protons from relative atomic mass. Example. Find quantity of protons in iron atom. To atom of iron there corresponds Fe chemical element (ferrum). Its relative atomic mass is equal to 56. Serial number of an element is equal to 26. Quantity of neutrons N=56-26=30.
2. For a certain isotope an additional explanation is always given. Before designation of an element its relative atomic mass and serial number is specified in the periodic table. In this case take the atomic mass specified in record of isotope. For example, usual oxygen has mass number 16 and serial number 8, quantity of neutrons in it N=16-8=8. Its stable isotope oxygen-18, has the corresponding mass number and quantity of neutrons in a kernel N=18-8=10.
3. Determine quantity of neutrons by the mass of a kernel and its charge. If weight is given in kilograms, divide it into number 1.661∙10^ (-27). As a result receive weight in atomic units of mass (relative atomic mass). Divide a kernel charge in pendants into number 1.6022 • 10^(-19) (a charge of one proton in pendants). It will be number of protons. When translating round all sizes to whole. Find number of neutrons, having taken away quantity of protons from relative atomic mass. Example. The mass of atom is equal to 11.627∙10^ (-27) to kg. The charge of its kernel makes 4.8•10^ (-19) C. Find the relative atomic mass of element 11.627∙10^ (-27) / (1.661∙10^ (-27)) =7. Calculate quantity of protons 4.8•10^ (-19) C of/(1,6022•10^ (-19))≈3. Define number of neutrons N=7-3=4.