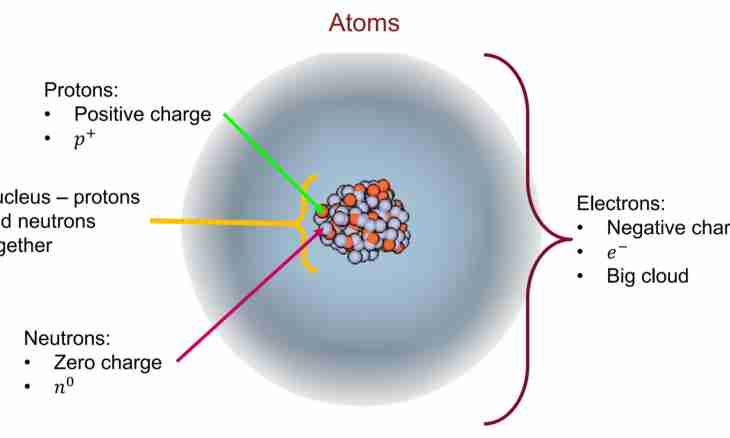
Atoms consist of subatomic particles — protons, neutrons and electrons. Protons represent positively charged particles which are in the center of atom, in its kernel. It is possible to calculate number of protons of isotope on atomic number of the corresponding chemical element.
Atom model
For the description of properties of atom and its structure the model, known as "Model of atom across Bohr" is used. According to it the structure of atom reminds the solar system — the heavy center (kernel) is in the center, and easier particles move on an orbit around it. Neutrons and protons form a positively charged kernel, and negatively charged electrons move around the center, being attracted to it by electrostatic forces.
Element call the substance consisting of atoms of one type, it is defined by number of protons in each of them. The element is given the name and a symbol, for example, hydrogen (H) or oxygen (O). Chemical properties of an element depend on number of electrons and, respectively, number of the protons which are contained in atoms. Chemical characteristics of atom do not depend on number of neutrons as neutrons have no electric charge. However their number affects stability of a kernel, changing atom lump.
Isotopes and number of protons
Isotopes call atoms of separate elements with various number of neutrons. These atoms chemically identical, however have different weight, also they differ in the ability to emit radiation. The atomic number (Z) is a serial number of chemical element in periodic Mendeleev's system, it is defined by number of protons in a kernel. Each atom is characterized by atomic number and mass number (A) which to equally total number of protons and neutrons in a kernel. The element can have atoms with various number of neutrons, but the quantity of protons remains invariable and is equal to number of electrons of neutral atom. To define how many protons contain in an isotope kernel, are enough to look at its atomic number. The number of protons is equal to number of the corresponding chemical element in the periodic table of Mendeleyev.
Examples
As an example it is possible to consider hydrogen isotopes. Hydrogen atoms with one proton and without neutrons are most spread in the nature. At the same time there are hydrogen isotopes with one or two neutrons, they have the corresponding names. However they have at all one proton that corresponds to serial number of hydrogen in the periodic table. Hydrogen isotope with one neutron and mass number 2 is called a deuterium or heavy hydrogen, it is stable. Tritium, hydrogen isotope with mass number 3 and two neutrons, is radioactive. It is called sometimes superheavy hydrogen, and a tritium kernel — a triton.