Relative molecular weight is the dimensionless size showing how much the mass of a molecule it is more than 1/12 mass of carbon atom. Respectively, the mass of atom of carbon is equal 12 units. It is possible to determine a relative molecular lot of chemical compound, having put the mass of atoms of which the substance molecule consists.
It is required to you
- - handle;
- - note paper;
- - calculator;
- - Mendeleyev's table.
Instruction
1. Write down a chemical formula of connection which relative molecular lot is required to be calculated. For example, orthophosphoric H3PO4 acid. From a formula you can see that molecule acids consists of three atoms hydrogen, one atom of phosphorus and four atoms of oxygen.
2. Find in Mendeleyev's table of a cell of elements of which this molecule consists. Values of relative atomic masses (Ar) for each substance are specified in the left bottom corner of a cell. Rewrite them, having rounded to an integer: Ar(H) – 1; Ar(P) – 31; Ar(O) – 16.
3. Determine a relative molecular lot of connection (Mr). For this purpose increase the atomic mass of each element by amount of atoms in a molecule. Then put the turned-out values. For orthophosphoric acid: Mr(н3ро4) = 3*1 + 1*31 + 4*16 = 98.
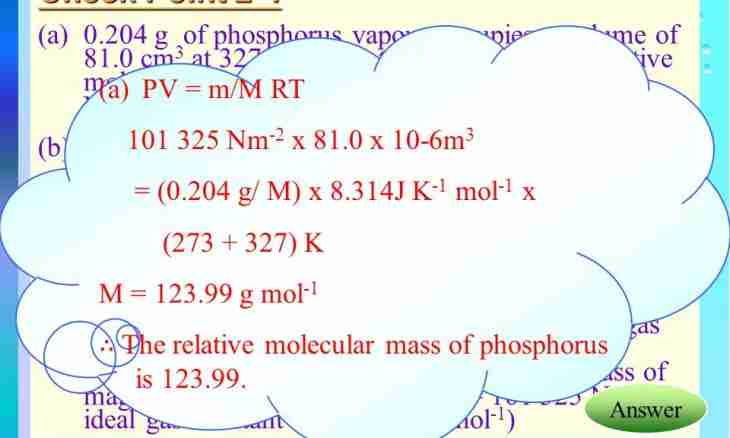
4. Relative molecular weight coincides with the molar mass of substance in number. In some tasks this communication is used. Example: gas at a temperature of 200 To and pressure of 0.2 MPas has density of 5.3 kg / mz. To determine its relative molecular weight.
5. Use Mendeleyev-Clapeyron's equation for ideal gas: PV = mRT/M, where V – the volume of gas, m3; m – the mass of this ate around gas, kg; M – molar mass of gas, kg/mol; R – universal gas constant. R=8.314472 m2kg with-2 Mol-1 K-1; T – gas temperature, To; P - absolute pressure, Pa. Express molar weight from this dependence: M = mRT / (PV).
6. It is known that density formula: p = m/V, kg/m3. Substitute it in expression: M = pRT/P. Determine the molar mass of gas: M = 5.3*8.31*200 / (2*10^5) = 0.044 kg/mol. Relative molecular mass of gas: Mr = 44. You can assume that it is carbon dioxide: Mr(CO2) = 12 + 16*2 = 44.