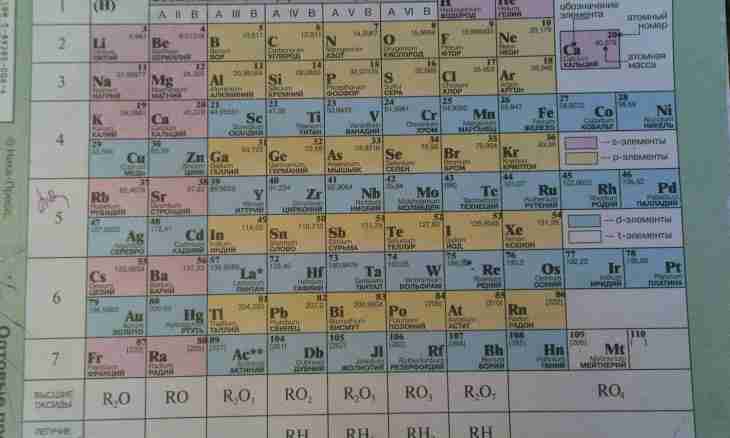
Dmitry Ivanovich Mendeleyev's table is a universal reference material by which it is possible to recognize the most necessary data on chemical elements. The most important is to know the basic principles of its "reading", that is it is necessary to be able to use correctly this information material that will serve as great help for the solution of any tasks in chemistry. Especially as the table is resolved on all types of control of knowledge, including even the USE.
It is required to you
- I. Mendeleyev's table, handle, paper
Instruction
1. The table represents structure in which chemical elements by the principles and laws are located. That is, one may say, that the table is multy-storey "house" in which there "live" chemical elements, and everyone them them has the own apartment at certain number. Across "floors" - the periods which can be small and big are located. If the period consists of two rows (that is specified sideways by numbering), then such period is called big. If it has only one row, then is called small.
2. Also the table is divided into "entrances" - groups which only eight. As are in any stairwell of the apartment at the left and on the right, and here chemical elements are located by the same principle. Only in this option their placement unevenly – on the one hand is more than elements and then speak about the main group, with another - it is less and it demonstrates that group collateral.
3. Valency is an ability of elements to form chemical bonds. There is a valency a constant which does not change also the variable having various value depending on that what part of substance the element is. When determining valency according to Mendeleyev's table it is necessary to pay attention to such characteristics: No. of group elements and its type (that is main or collateral group). Constant valency in this case is identified by number of group of the main subgroup. To learn value of variable valency (if that is, and, usually at nonmetals), then it is necessary from 8 (only 8 groups – from here such figure) to subtract No. of group in which the element is located.
4. Example No. 1. If to look at elements of the first group of the main subgroup (alkaline metals), then it is possible to draw a conclusion that all of them have the valency equal to I (Li, Na, To, Rb, Cs, Fr).
5. Example No. 2. Elements of the second group of the main subgroup (alkaline-earth metals) respectively have the valency of II (Be, Mg, Ca, Sr, Ba, Ra).
6. Example No. 3. If to speak about nonmetals, then for example, P (phosphorus) is in the V group of the main subgroup. From here its valency will be equal to V. Besides phosphorus has one more value of valency, and for its definition it is necessary to perform operation 8 - No. of an element. Means, 8 – 5 (number of group of phosphorus) = 3. Therefore, the second valency of phosphorus is equal to III.
7. Example No. 4. Halogens are in the VII group of the main subgroup. Means, their valency will be equal to VII. However considering that it is nonmetals, it is necessary to make arithmetic action: 8 – 7 (No. of group of an element) = 1. Therefore, other valency of halogens is equal to I.
8. For elements of secondary subgroups (and only metals concern them) the valency needs to be remembered especially as a case it is equal in the majority to I, II, are more rare than III. Also it is necessary to learn valencies of chemical elements which have more than two values.