Action of osmotic pressure corresponds to the well-known principle of Le Chatelie and the second law of thermodynamics: the biological system in this case seeks to level concentration of substances in solution in two environments which are divided by a semipermeable membrane.
What is the osmotic pressure
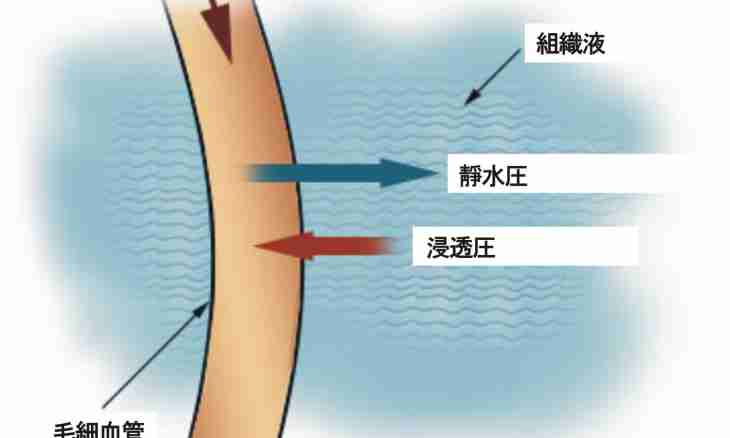
Understand the hydrostatic pressure which influences solutions as osmotic pressure. At the same time liquids have to be divided by a semipermeable membrane. In similar conditions through a membrane the processes of diffusive dissolution do not go.
Semipermeable call membranes which permeability is high only for certain substances. The film which adjoins to an egg shell from within can be an example of a semipermeable membrane. It detains sugar molecules, but does not obstruct water motion of the molecules.
The purpose of osmotic pressure – creation of balance between concentration of two solutions. Molecular diffusion between solvent and the dissolved substance becomes means of achievement of such purpose. In records pressure of this type is usually designated by the letter "p".
The phenomenon of osmosis takes place in those environments where mobile properties of solvent exceed those at the dissolved substances.
Properties of osmotic pressure
Osmotic pressure is characterized by property of a tonichnost which is considered its gradient measure. It is about the potential of difference between couple of solutions which are separated from each other by a membrane of semipermeable type.
Substance which in comparison with other solution has more essential indicator of osmotic pressure is called hypertensive solution. Hypotonic solution has a low indicator of osmotic pressure. Place similar solution in the closed space (for example, in a blood cage) – and you will see how osmotic pressure will break off a cell membrane.
When enter drugs into blood, they right at the beginning mix up with isotonic solution. That the osmotic pressure of cellular liquid was balanced, sodium chloride in solution has to contain in a certain proportion. If drugs made on the basis of water, osmotic pressure would destroy blood cages. During creation of solutions with high concentration of substances water will be forced to leave cages – as a result they will begin to contract.
Unlike animal cages, in vegetable under the influence of pressure their contents are exposed to a separation from a cover. This phenomenon is called plazmolizy.
Interrelation between solution and osmotic pressure
The chemical nature of the substances which are contained in solution does not influence the size of osmotic pressure. This indicator is defined by amount of substance in solution. Osmotic pressure will begin to grow with increase in solution of active agents.
So-called onkotichesky osmotic pressure depends on amount of the proteins which are contained in solution. At long starvation or a disease of kidneys the level of concentration of proteins in an organism goes down. Water from fabrics passes into vessels.
Condition for creation of osmotic pressure are existence of a semipermeable membrane and presence from two of its parties of solutions. At the same time their concentration has to be various. The cellular membrane is capable to pass parts of certain sizes: for example, through it there can pass the water molecule.
If to use the special materials having ability of division it is possible to separate from each other components of mixes.
Value of osmotic pressure for biological systems
If the biological structure contains a partition of semipermeable type (fabric or cellular cover), then continuous osmosis will begin to create excessive hydrostatic pressure. There is possible a hemolysis at which there is a rupture of a cell membrane. Opposite process is observed if to place a cage in the concentrated salt solution: the water which is contained in a cage will get through a membrane into salt solution. Wrinkling of a cage will become result, it loses a stable state.
As the membrane of a pronitsayem only for particles of a certain size, it is capable to pass substances selectively. Let's say water passes through a membrane freely whereas molecules of ethyl alcohol do not manage to make it.
Examples of the simplest membranes through which there passes water, but there do not pass many other substances dissolved in water, can serve:
- parchment;
- skin;
- specific fabrics of plant and animal origin.
The mechanism of osmosis is defined in organisms of animals by the nature of membranes. Sometimes the membrane functions by the principle of a sieve: detains big particles and does not obstruct the traffic of small. Molecules only of certain substances are capable to pass in other cases through a membrane.
Osmosis and the related pressure play extremely important role in development and functioning of biological systems. Constant transfer of water in cellular structures provides elasticity of fabrics and their durability. Processes of digestion of food and a metabolism are connected with differences in permeability of fabrics for water directly.
Osmotic pressure serves as the mechanism by means of which in cages nutrients move. At high trees biologically active elements at the expense of osmotic pressure rise by height to several dozen meters. Extreme height of plants in terrestrial conditions is defined including by indicators which characterize osmotic pressure.
Soil moisture together with nutrients moves in plants thanks to the osmotic and capillary phenomena. Osmotic pressure in plants can reach 1.5 MPas. Lower indicators of pressure have roots of plants. Increase in osmotic pressure from roots to leaves is extremely important for the movement of juice on a plant.
Osmosis regulates water supply in cages and intercellular structures. Thanks to osmotic pressure quite certain form of bodies remains.
Biological liquids of the person are water solutions of low-molecular and high-molecular connections, polysaccharides, proteins, nucleinic acids. Osmotic pressure in a system is defined by cumulative action of these components.
Treat biological liquids:
- lymph;
- blood;
- fabric liquids.
At medical procedures it is necessary to use solutions which contain the same components which are included in composition of blood. And in the same quantities. Solutions of this kind found broad application in surgery. However in significant amounts it is possible to enter into blood of the person or animals only isotonic solutions, that is those which reached balance.
At 37 degrees Celsius the osmotic pressure of human blood is about 780 kPa that corresponds to 7.7 atm. Admissible and harmless fluctuations of osmotic pressure are insignificant and even in case of heavy pathology do not exceed some minimum values. It is explained by the fact that the human body is characterized by a homeostasis – constancy of the physical and chemical indicators influencing activity.
Osmosis is widely applied in medical practice. In surgery use hypertensive bandages long ago and successfully. The gauze moistened in hypertensive solution helps to cope with purulent wounds. According to the law of osmosis, liquid from a wound goes outside. As a result the wound is constantly cleaned from disintegration products.
Kidneys of the person and animals can be a good example of "the osmotic device". Exchange products come to this body from blood. Water and the smallest ions which return through a membrane to blood gets into urine from kidneys by means of osmosis.