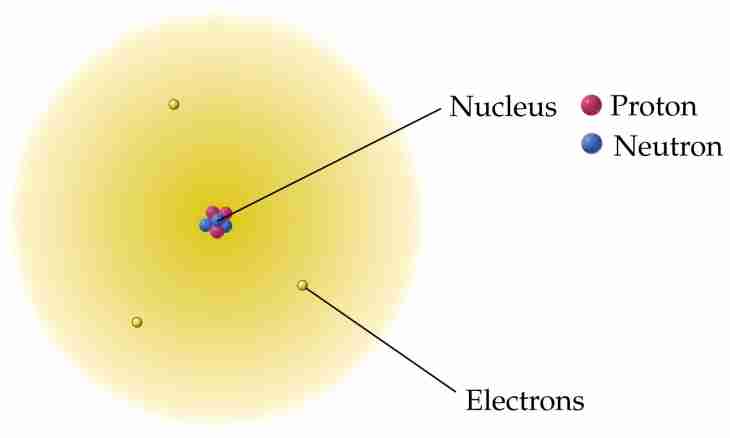
Attraction force between an atomic nucleus of hydrogen and an electron which is located in an orbit of this atom can be found, proceeding from knowledge of physics of interaction of these particles with each other.
It is required to you
- The textbook in physics of the 10th class.
Instruction
1. Using the textbook in physics of the 10th class, sketch on a leaf that represents hydrogen atom. It is known that this chemical element contains in the kernel only one proton around which one electron rotates.
2. Pay attention that subatomic particles of hydrogen have heteronymic charges. This circumstance leads to the fact that the proton and an electron are attracted to each other with some force.
3. Write out from the textbook as the Coulomb force of interaction of charges is defined. This type of interaction is inherent in electron attraction force with a kernel. It is known that the module of Coulomb force of interaction is directly proportional to the work of charges of the interacting particles and is inversely proportional to a distance square between these particles. The coefficient of proportionality is called an electric constant.
4. Define, using the tables of constants located at the end of the textbook what the electric constant is equal to. Substitute its value in a formula for Pendant force.
5. Find in the textbook in physics the table of characteristic sizes of some particles. Determine by this table of weight and charges of an electron and a proton.
6. Take as approximate value of distance between an electron and a proton the size of a half of an angstrom. One angstrom is equal to ten in minus of the tenth degree meters. Substitute all necessary sizes in expression for the Coulomb force of an attraction and calculate its value.
7. Remember that all material bodies are also attracted to each other with a force of a gravitational attraction. The formula for this force is similar to expression for Pendant force. The difference is only that instead of the work of charges in expression of gravitational force costs the work of masses, and as coefficient of proportionality the gravitational constant is used.
8. Substitute sizes of masses, distances and a gravitational constant in a ratio of gravitational force of an attraction and calculate the size of this force. Put the gravitational force of an attraction with Coulomb. The received value will be to equally full force of an attraction of an atomic nucleus of hydrogen and an electron.