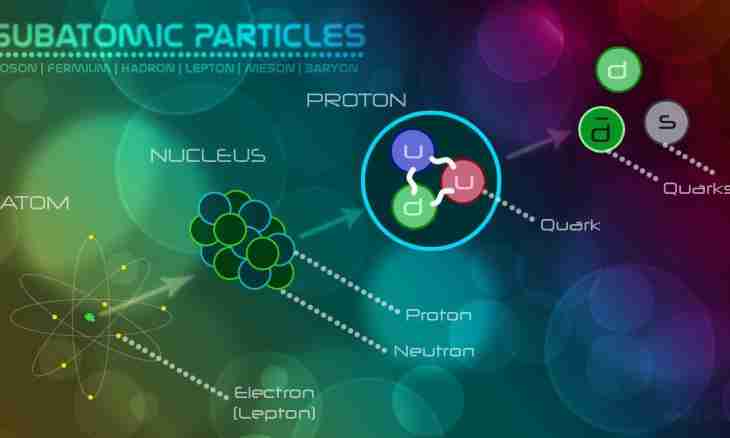
Atom submits itself kind of the tiny copy of the Solar system. Only instead of the Sun in its center the massive kernel is located, and instead of planets elementary particles - electrons rotate. Atom electrically is neutral therefore the total negative charge of electrons has to be counterbalanced by a total positive charge of the same size. It occurs as the kernel consists of other elementary particles – protons and neutrons. Each proton bears the same charge in size as an electron, only with the opposite sign.
Instruction
1. Let's say under the terms of a task the total positive charge Q atomic nuclei is known to you. To learn quantity of protons of N, it is necessary to divide Q into qpr proton charge size only. (she can be recognized in the reference book on physics or chemistry). That is N = Q/qpr.
2. It is possible to find quantity of protons in a kernel by means of Mendeleyev's table. In this table concrete strictly certain place depending on its chemical properties is allocated to each element. And chemical properties, first of all, are defined by the structure of atom of an element.
3. Necessary data on chemical element, including its serial number are provided in each cell of the table. Here it just corresponds to quantity of protons in an element atomic nucleus.
4. Look in the table. An element at number 11 – alkaline metal sodium (Na). Therefore, in each atom of sodium there are 11 protons. Or an element at number 23 – metal the vanadium (V) showing in the connections not only the main but also acid properties. Proceeding their serial number, it is possible to draw a conclusion: in each atom of vanadium there are 23 protons.
5. Carry out a peculiar inspection. It is known that atom electrically is neutral. But as neutrons do not bear charges at all, only electrons can counterbalance a total positive charge of protons of atom. Means, in atom of sodium there have to be 11 electrons, and in vanadium atom – 23. Check, whether so it.
6. Again take Mendeleyev's table. Are provided the data showing how electronic levels of atom of this element are filled in each cell. At sodium: two electrons at the first level, eight electrons at the second level and one on third (external). In the sum eleven electrons. At vanadium: two electrons at the first level, eight – on the second, eleven – on the third and two – on the fourth (external). In the sum twenty three electrons. Total charges are counterbalanced, atoms electrically are neutral.