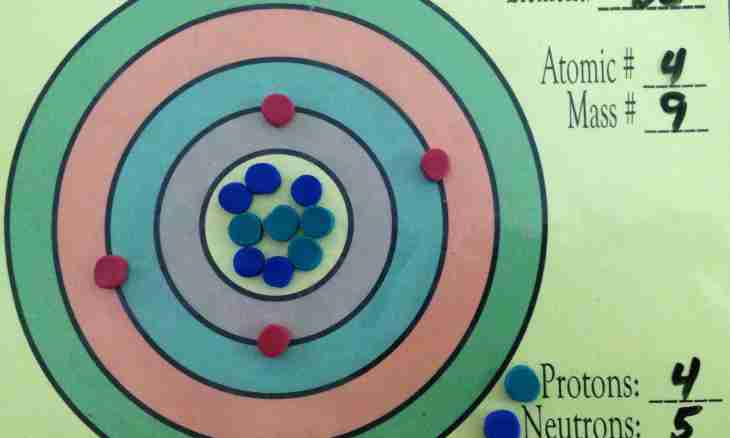
According to the standard model, atomic nuclei of any chemical element consist of protons and neutrons. These smallest particles were open at different times. Each of opening on a step brought closer scientists to use of nuclear power.
Opening of a proton
The proton is an atomic nucleus of hydrogen, an element which has the simplest building. It has a positive charge and almost unlimited time of life. It is the most stable particle in the Universe. The protons formed as a result of the Big Bang still did not break up. The mass of a proton is 1.627*10-27 kg or 938.272 ev. More often this size is expressed in electronvolts.
The proton was opened by "father" of nuclear physics Ernest Rutherford. He made a hypothesis that atomic nuclei of all chemical elements consist of protons as on weight they exceed a hydrogen atomic nucleus in an integer of times. Rutherford conducted useful experience. In those days the natural radioactivity of some elements was already open. By means of the alpha radiation (alpha particles represent helium kernels with high energy) the scientist irradiated nitrogen atoms. As a result of such interaction the particle took off. Rutherford assumed that it is a proton. Further experiments in the bubble camera of Wilson confirmed his assumption. So in 1913 the new particle was open, but Rutherford's hypothesis of structure of a kernel was insolvent.
Opening of a neutron
The great scientist found a mistake in the calculations and made a hypothesis of existence of one more particle which is a part of a kernel and having practically the same weight as a proton. Experimentally he could not find it. Made it in 1932 the English scientist James Chadwick made. He conducted an experiment during which bombarded beryllium atoms high-energy alpha particles. As a result of nuclear reaction from a kernel of beryllium the particle subsequently called a neutron took off. For the opening Chadwick in three years got the Nobel Prize. The mass of a neutron really differs from the mass of a proton (1.622*10-27 kg) a little, but this particle does not possess a charge. In this sense she is neutral and at the same time capable to cause division of heavy-nuclei. Due to the lack of a charge the neutron can easily pass through a high Coulomb potential barrier and take root into structure of a kernel. The proton and a neutron have quantum properties (can show properties of particles and waves). Neutron radiation is used in the medical purposes. The high penetration allows this radiation to ionize deep tumors and other malignancies and to find them. At the same time energy of particles rather small. A neutron, unlike a proton, an unstable particle. Its time of life is about 900 seconds. It breaks up to a proton, an electron and an electronic neutrino.