The molecular mass of substance is the mass of a molecule expressed in atomic units and in number equal to molar weight. When calculating in chemistry, physics and the equipment the calculation of values of molar mass of various substances is often used.
It is required to you
- - Mendeleyev's table;
- - table of molecular masses;
- - table of values of the cryoscopic constant.
Instruction
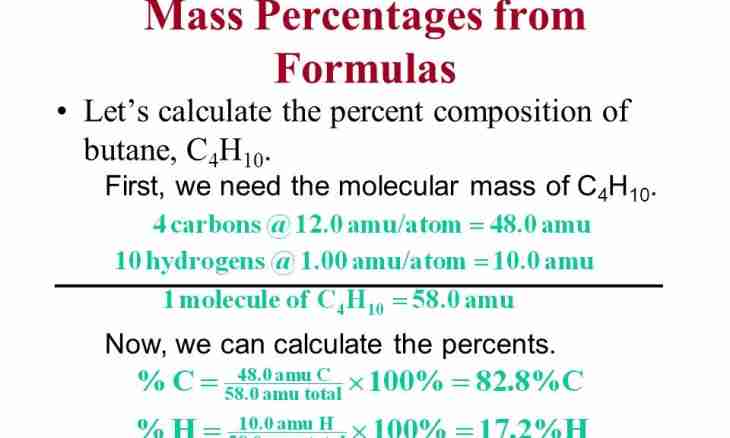
1. Find the necessary element in Mendeleyev's table. Pay attention to fractional numbers under its sign. For example, oxygen O has in a cell the numerical value equal 15.9994. It is the atomic mass of an element. Atomic mass needs to be increased by the index of an element. The index shows how many molecules of an element contain in substance.
2. If complex substance is given, increase the atomic mass of each element by its index (if one atom of any given element contains and there is no index respectively, multiply by unit) and put the received atomic masses. For example, the molecular mass of water is calculated so - MH2O = 2 MH + by MO ≈ 2·1+16 = 18 and. e. m.
3. Calculate molecular weight from the special table of molecular masses. Find tables in the Internet or get their printing option.
4. Calculate molar weight by means of suitable formulas and equate it to molecular. Replace units of measure from g/mol on and. e. m. If pressure, volume, temperature on an absolute scale of Calvin and weight is given, calculate the molar mass of gas on Mendeleyev-Clapeyron's equation of M= (m∙R∙T) / (P∙V) in which M - molecular (molar weight) in and. e. the m, R is a universal gas constant.
5. Calculate molar weight on a formula M=m/n where m is the mass of any this substance, n is chemical amount of substance. Express amount of substance through Avogadro's number of n=N/NA or by means of n=V/VM volume. Substitute in a formula above.
6. Find the molecular mass of gas if only the value of its volume is given. For this purpose take a tight cylinder of the known volume and pump out from it air. Weigh it on scales. Pump gas in a cylinder and again measure weight. The difference of mass of a cylinder with the gas pumped in it and an empty cylinder is the mass of this gas.
7. By means of the manometer find pressure in a cylinder (in Pascals). The thermometer take ambient temperature, it is equal to temperature in a cylinder. Transfer degrees Celsius to Kelvins. For this purpose to the received value add 273. Find molar weight on Mendeleyev-Klapeyrona's equation given above. Transfer it to molecular, having replaced units of measure on and. e. m.
8. If it is necessary to carry out a krioskopiya, calculate molecular weight from formula M = Р1∙Ек∙1000/Р2Δtk. The P1st P2 is the mass of the dissolved substance and solvent respectively, in grams, Ek is the cryoscopic constant of solvent (learn it from the table, it is various for different liquids); Δtk — the difference of temperatures measured by means of the metastatic thermometer.