Benzene is aromatic hydrocarbon which cornerstone the group of atoms of carbon connected among themselves cyclically is. And this special group is called a benzene ring, or an aromatic kernel.
Specifics of the structure of benzene
In far 1825, Michael Faraday the English scientist, investigated vorvan. At its thermal decomposition, substance with a strong smell was emitted. Its molecular formula was C6H6. This connection is called the most plain aromatic hydrocarbon, or benzene today.
The structural formula offered by already German chemist Kekule in 1865 became widespread. It represents the alternating unary and double communications between carbon atoms, becoming isolated in a ring. When Kekule worked on this subject, he dreamed about a snake that bit herself a tail. Thanks to this dream he managed to create a benzene ring structurally, defined the spatial provision of atoms of carbon rather each other.
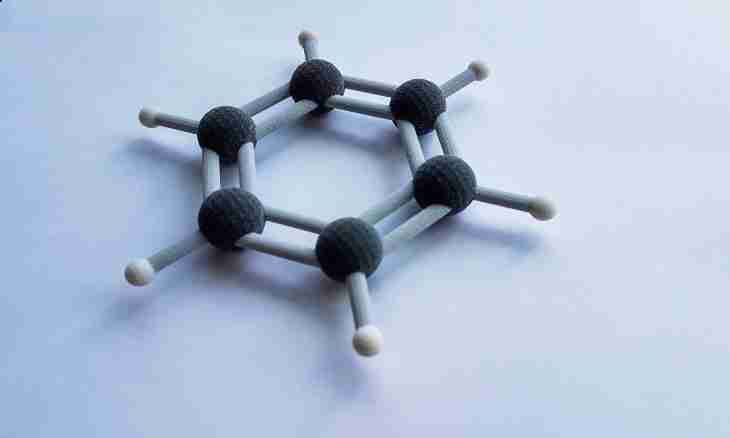
In a benzene molecule the habitual unary and double communications between atoms of carbon are absent, they are equally equivalent, represent intermediate, so-called one-and-a-half, communications. With their help an educated only benzene ring, in other substances this type of communication does not meet. Feature of a benzene ring is that all atoms which are a part of this substance are in one plane, and the framework it is formed by carbon atoms, creating the correct hexagon. All valent corners make 120 degrees, they are equal.
Benzene orbitals
Each atom of carbon in a molecule of benzene has identical electronic density. A condition of each of them – sr2-hybridization. From this it is visible that only three orbitals, one – s, and two – river are hybridized. One r-orbital remains not hybrid. Two hybrid r-orbitals are blocked with two adjacent atoms of carbon, the s-orbital of hydrogen is blocked with the third orbital. Not hybrid r-orbital has the dumbbell form, it is located at an angle in 90 degrees to a s-orbital. As a result at benzene the r-orbital of each atom of carbon is blocked with two adjacent similar r-orbitals of atoms, it turns out that adjacent electrons among themselves interact, form the r-electronic cloud uniform for all atoms. Graphically it is represented in the form of a ring in the correct hexagon.
Physical and chemical properties of benzene
Benzene with the homologs is represented by specifically smelling liquids not having color. Their specific weight is less, than at water, in it they are not dissolved, but in organic liquids, such as acetone, air and alcohol, dissolved easily. Durability at a benzene kernel very high, thanks to it it easily enters replacement operations. Hydrogen atoms in a kernel are very mobile, for this reason of reaction of sulphonation, halogenations, nitrations proceed rather easily.